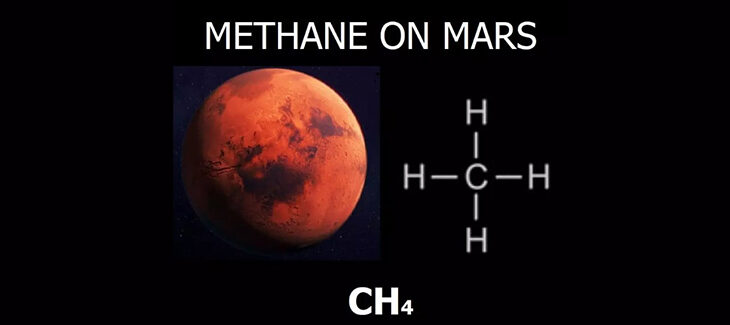
The ExoMars Trace Gas spacecraft began studying the atmosphere of Mars, with a particular focus on trace gases such as methane. Despite the minimal presence of methane in the total volume of the atmosphere, it is a crucial element for understanding the current activity of the planet.
Credit: ESA/ATG medialab
The illustration below demonstrates various potential methods for adding or removing methane from the atmosphere. One promising scenario involves the production of methane by microorganisms. This gas can then be buried below the surface and stored in icy, network-structured forms known as clathrates for later release into the atmosphere.
Methane can also be formed during reactions between water and olivine-bearing rocks, possibly under conditions of increased volcanic activity. Likewise, the gas may be stored beneath the earth’s surface in ice structures and released into the atmosphere through surface cracks or volcanoes.
Ultraviolet rays can break down methane molecules and cause them to form through reactions with other molecules or organic substances already on the surface, such as dust from comets reaching Mars.
Methane can also move quickly across the planet’s surface due to strong winds, which “splits” its signal and makes identifying specific sources more difficult.
Methane on Mars is thought to have a relatively short half-life of about 400 years, implying that any methane reserves discovered must have been created or released relatively recently. The Trace Gas Orbiter spacecraft will gradually map the distribution of methane to analyze its geographic and seasonal distribution and ultimately identify where it is released or produced.
The device can detect and analyze methane and other gases even in low concentrations, providing accuracy that is three orders of magnitude higher than previous measurements. In addition, it can detect key “isotopologues” of methane and water (isotopologues are molecules that have at least one atom with a different number of neutrons compared to the original chemical element) to help distinguish between different scenarios for their formation.
Banner image: Geo Forward
Image credit:
https://www.geoforward.com
https://www.esa.in