Protons are subatomic particles that form the nucleus of an atom together with neutrons and are not elementary but consist of quarks. With their help, atoms are built from which the universe is formed.
Hydrogen, the lightest element, has a single proton nucleus. The heaviest element on the periodic table, Oganesson, has 118 protons.
If you blow up a hydrogen atom to the size of your bedroom, the proton will be the size of a grain of dust so small that it will be very difficult to notice. Precisely because the proton is so small, we can ignore the chaos going on inside it, describing the hydrogen atom as simple. More precisely, the size of a proton is 100,000 times smaller than the size of a hydrogen atom.
For comparison, the size of the Sun is only 3000 times smaller than the size of the solar system, if you count along the orbit of Neptune. That’s right – the atom is more empty than the solar system!
Chaos inside the proton
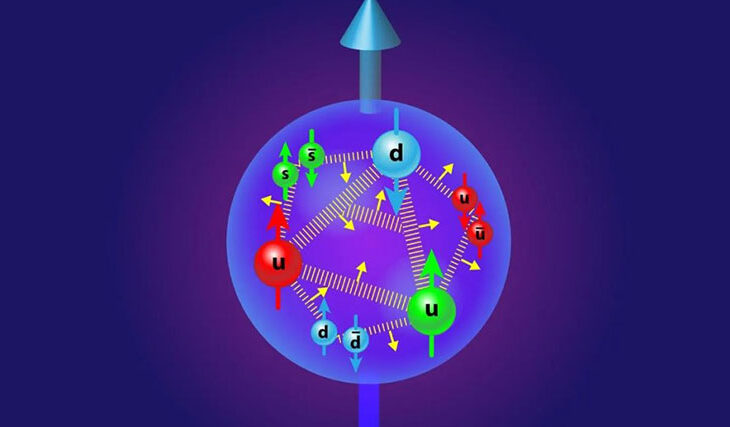
Protons are tiny particles only a femtometer in diameter. They are made up of even smaller particles called quarks. Like neutrons, protons contain three quarks (two “up” quarks and one “down” quark) that are held together inside the proton by “strong force”. But, in the proton, there are a myriad of gluons (they are the majority), antiquarks, and quarks. Two top and one bottom scientists is an expression that scientists use to describe the “naive” model of the proton.
A whole bunch of quarks, antiquarks, and gluons (there are too many of them and they change too quickly to be practically counted) rush about at tremendous speeds inside the proton.
In 1967, an important experimental study was carried out at the Stanford Linear Accelerator Center (SLAC) that confirmed the existence of quarks inside the proton. Earlier research involved throwing electrons at protons and seeing them ricochet, similar to the impact of billiard balls. However, SLAC managed to eject electrons with much higher energy, which allowed scientists to observe deviations that differed from previous observations.
These high-energy electrons, when colliding with protons, hit them so hard that they lead to the destruction of the proton, which is called deep inelastic scattering. In this case, the electrons bounced off the smallest particles inside the proton, which were called quarks. This experiment was the first convincing proof of the existence of quarks and confirmed their role inside the proton.
Figuratively, we can say that the proton looks like a dandelion.
Discovery of protons
British physicist Ernest Rutherford questioned the plum pudding model, in which atoms were thought to be uniformly scattered with a positive charge.
Geiger and Marsden experimented with alpha particles and a sheet of gold that deflected at large angles or bounced back, indicating the presence of a small, dense nucleus in the atom. This model called the Bohr model, states that an atom consists of a dense nucleus surrounded by space where electrons spin. Rutherford and Niels Bohr concluded that the hydrogen nucleus, called the proton, is the basic building block of all atomic nuclei.
What is the charge of a proton?
The proton has an elementary charge, denoted as “e”, which serves as the basic measure of charge used to measure all other electric charges. The only particles with less charge than the proton are quarks.
The elementary charge of a proton is 1.602192 x 10-19 coulombs (C). This charge is positive and exactly equal, but opposite in sign, to the electron’s charge, which is -1.602192 x 10-19 coulombs.
Since the charges of the proton and electron are equal, and the neutron, the neighbor of the proton in the atomic nucleus, is a charge-neutral particle, atoms remain electrically neutral if the number of protons and electrons in them is the same. However, if an electron is removed from an atom, this will upset the balance of charges between electrons and protons, making the atom a positively charged ion.
What is the size and mass of a proton?
Protons are so small that their size corresponds to only one femtometer (10-15 meters). This is approximately one ten billionths of the radius of an atom (10-10 meters). The thickness of a human hair, for example, is 10-8 meters, i.e. the proton is seven orders of magnitude smaller.
Despite their tiny size, protons have an impressive mass of 1.673 x 10-27 kilograms, which is 1836 times heavier than the mass of an electron (9.1 x 10-31 kilograms) and slightly less than the mass of a neutron (a neutron has a mass of 1.008 times the mass proton).
Image credit:
https://www.forbes.com
https://www.mpg.de